Gut Cell Atlas Crohn’s Disease Consortium

This consortium of grantees aims to generate a Gut Cell Atlas for Crohn’s disease by cataloging the many cell types in the small and large intestines with single-cell technologies. It is part of the pathbreaking global Human Cell Atlas effort, which is transforming what we know about cells and their function in human health.
The Helmsley Charitable Trust is focused on finding a cure for Crohn’s disease. This is a long-term pursuit, so in parallel we are dedicated to improving patients’ lives today.
The creation of a Crohn's Disease Gut Cell Atlas, with Helmsley support, will offer tremendous potential to achieve both goals. It will enable better understanding of the disease and spur new, more personalized treatments on the path to prevention and a cure – not just for Crohn’s disease, but for improving gut health overall.
Atlasing projects focus on a variety of single-cell analyses to build a Crohn’s Disease Gut Cell Atlas, from broad, comprehensive sequencing to cell type-specific efforts. Samples from both Crohn’s disease patients and healthy individuals are obtained, and transcriptomic, proteomic, and spatial data are generated.
Pilot projects develop or test novel technologies that aim to elucidate cellular behavior that leads to intestinal inflammation. Support grants provide added tools and resources to the many activities of the Crohn’s Disease Gut Cell Atlas Consortium to maximize its impact and success.
Past Collaborations include grants that are part of the Gut Cell Atlas Crohn’s Disease Consortium efforts and are now completed.
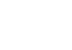
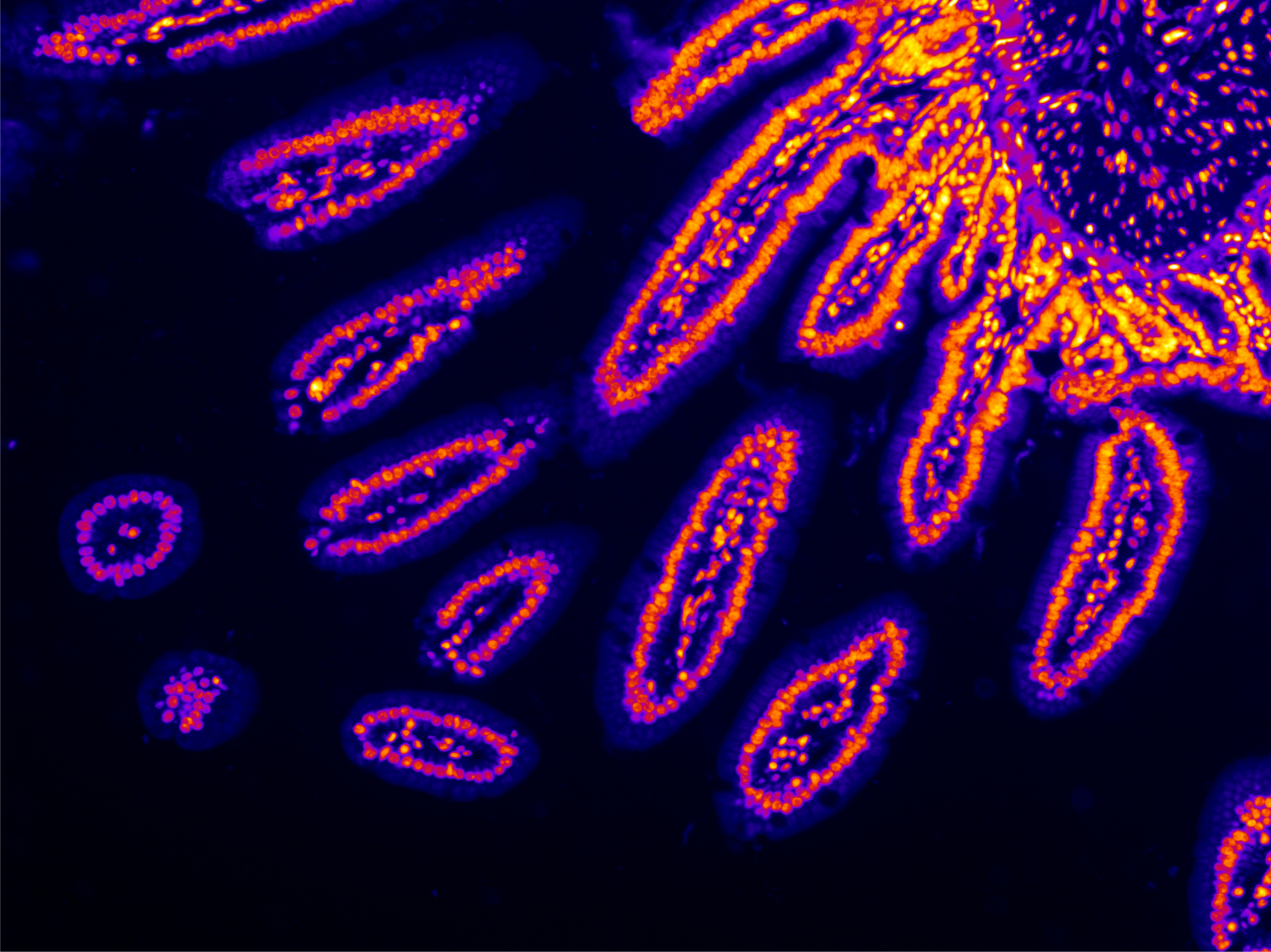
 The goal of this study is to deliver a spatially resolved atlas of the terminal ileum to broaden our understanding of pediatric Crohn’s disease using state-of-the-art single-cell methods with a unique treatment-naïve patient cohort. If successful, this project will lead to a greater understanding of the cellular changes that occur with disease, especially in treatment naïve patients, which may lead to potential new targets for improved treatments for pediatric patients.
The goal of this study is to deliver a spatially resolved atlas of the terminal ileum to broaden our understanding of pediatric Crohn’s disease using state-of-the-art single-cell methods with a unique treatment-naïve patient cohort. If successful, this project will lead to a greater understanding of the cellular changes that occur with disease, especially in treatment naïve patients, which may lead to potential new targets for improved treatments for pediatric patients.
Team: Jose Ordovas-Montanes, Leslie Kean, Scott Snapper
 “The Gut Cell Atlas, as well as the Human Cell Atlas, represent one of the forefronts of scientific research, as we try to understand complex biological systems by examining the activities and interactions of large numbers of individual cells. Studies at such a single-cell level will provide deeper understanding and generate new ideas for disease prevention and treatments.”
“The Gut Cell Atlas, as well as the Human Cell Atlas, represent one of the forefronts of scientific research, as we try to understand complex biological systems by examining the activities and interactions of large numbers of individual cells. Studies at such a single-cell level will provide deeper understanding and generate new ideas for disease prevention and treatments.”
The goal of this study is to generate a longitudinal single-cell map of the terminal ileum and ascending colon for treatment-naïve pediatric Crohn’s disease, ultimately to observe what changes occur in inflammation and with treatment. This project will also focus on developing novel computational methods for automated cell type interpretation and single-cell analysis.
Team: Subra Kugathasan, Greg Gibson, Eliver Ghosn
Publications
Domain adaptation for supervised integration of scRNA-seq data. Communications Biology (2023).
Quantifying the clusterness and trajectoriness of single-cell RNA-seq data. PLOS Computational Biology (2024).
Recursive clustering of cellular diversity in scRNA-seq data. Journal of Computational Biology (2025).
Altered inflammatory mucosal signatures within their spatial and cellular context during active ileal Crohn’s disease. JCI Insight (2025).
 “The Gut Cell Atlas is part of an exciting push to catalog all the cells that make up the human body. Although only one step on the way to understanding human intestinal biology and disease, it is an important one. The Atlas will provide an essential knowledge base for understanding how ‘things go wrong’ in inflammatory bowel diseases.”
“The Gut Cell Atlas is part of an exciting push to catalog all the cells that make up the human body. Although only one step on the way to understanding human intestinal biology and disease, it is an important one. The Atlas will provide an essential knowledge base for understanding how ‘things go wrong’ in inflammatory bowel diseases.”
The goal of this study is to generate a comprehensive atlas of specialized immune tissues in healthy and Crohn’s disease terminal ileum and ascending colon. The investigators aim to develop methods to map cell interactions and elucidate immune cell development and trafficking in the intestinal immune response in health and Crohn’s disease.
Team: William W. Agace
Publications
The small and large intestine contain related mesenchymal subsets that derive from embryonic Gli1+ precursors. Nature Communications (2023).
Human gut-associated lymphoid tissues (GALT); diversity, structure, and function. Mucosal Immunology (2021).
Identification, isolation and analysis of human gut-associated lymphoid tissues. Nature Protocols (2021).
 “We will generate datasets that will be of use to researchers around the world for the benefit of Crohn’s disease patients.”
“We will generate datasets that will be of use to researchers around the world for the benefit of Crohn’s disease patients.”
The goal of this study is to provide an integrated dataset of single-cell gene expression data mapped onto a high-resolution 3-dimensional framework from intestines (terminal ileum and ascending colon) of healthy individuals and Crohn’s disease patients undergoing resection due to intestinal obstruction or stricturing disease. This grant will also provide a gut-centric coordinate framework to map anatomical locations of cells providing novel insights into Crohn’s disease mechanisms, crucial to developing personalized therapeutic strategies, and new analytical tools for querying the GC database.
Team: Shahida Din, Richard Baldock, Albert Burger, Irene Papatheodorou, David Adams
Publications
Towards a clinically-based common coordinate framework for the human gut cell atlas: the gut models. BMC Medical Informatics and Decision Making (2023).
The Comparative Pathology Workbench: Interactive visual analytics for biomedical data. Journal of Pathology Informatics (2023).
The Promise of Single-Cell RNA Sequencing to Redefine the Understanding of Crohn’s Disease Fibrosis Mechanisms. Journal of Clinical Medicine (2023).
The comparative pathology workbench: An update. Journal of Pathology Informatics (2025).
 “Our hope is that the Gut Cell Atlas will give us the capacity to work one cell at a time so we can develop completely new insights into the biological basis for Crohn’s disease. This should lead to out-of-the-box thinking, and thus new ways to address this debilitating disorder.”
“Our hope is that the Gut Cell Atlas will give us the capacity to work one cell at a time so we can develop completely new insights into the biological basis for Crohn’s disease. This should lead to out-of-the-box thinking, and thus new ways to address this debilitating disorder.”
The goal of this study is to construct an atlas of the terminal ileum and ascending colon containing single-cell gene expression and spatial information from biopsy and surgical samples from both healthy individuals and Crohn’s disease patients with varying degrees of inflammation. This grant will also provide tools to integrate the data from the multi-modality analysis of terminal ileal and colonic tissues, and related clinical data, including demographics and medications to correlate inflammation on the cellular level with patient history, symptoms, and response to medications.
Team: Lori Coburn, Qi Liu, Ken Lau, Bennett Landman, Gregor Neuert
Publications
A cross-platform informatics system for the Gut Cell Atlas: integrating clinical, anatomical and histological data. SPIE Medical Imaging (2021).
Cross-scale multi-instance learning for pathological image diagnosis. Medical Image Analysis (2024).
Identification and multimodal characterization of a specialized epithelial cell type associated with Crohn’s disease. Nature Communications (2024).
 The goal of this project is to build clinically useful predictors of response to biologic therapies for Crohn’s disease patients by measuring gene expression at single-cell resolution in blood samples and gut biopsies ascertained before and after drug initiation and combining these data with microbiome and metabolome data to build predictive models of treatment response. By identifying genetic variants associated with gene-expression levels they will identify genes, cell types, and pathways causally driving biologic drug response in inflammatory bowel disease. This project will pave the way to personalized medicine in Crohn’s disease and provide new insight into disease mechanisms.
The goal of this project is to build clinically useful predictors of response to biologic therapies for Crohn’s disease patients by measuring gene expression at single-cell resolution in blood samples and gut biopsies ascertained before and after drug initiation and combining these data with microbiome and metabolome data to build predictive models of treatment response. By identifying genetic variants associated with gene-expression levels they will identify genes, cell types, and pathways causally driving biologic drug response in inflammatory bowel disease. This project will pave the way to personalized medicine in Crohn’s disease and provide new insight into disease mechanisms.

 “These novel single-cell multimodal omics assays provide unprecedented opportunities to study complex cellular and molecular processes at single-cell resolution, and we are excited to deploy them to help achieve the goals of the Gut Cell Atlas initiative.”
“These novel single-cell multimodal omics assays provide unprecedented opportunities to study complex cellular and molecular processes at single-cell resolution, and we are excited to deploy them to help achieve the goals of the Gut Cell Atlas initiative.”
The goal of this study is to optimize a massively parallel, single-cell multimodal omics (transcriptome, cell surface protein epitope, and/or chromatin accessibility) assay for comparison of atlases of immune cells and associated molecular pathways across the spectrum of mucosal inflammation in patients with Crohn’s disease.
Team: Wei Chen
Publications
A unified model-based framework for doublet or multiplet detection in single-cell multiomics data. Nature Communications (2024).
The goal of this project is to develop an administrative core and data repository for the GCA Consortium to enable pre-publication collaboration and sharing between GCA grantees, as well as provide general support to the activities of the GCA.
Team: Bennett Landman, Deborah Hunter, Joseph Roland
Publications
OpenGCA: Gut cell Atlas Code Repository (2023).
The goal of this project is to plan, coordinate, and track the Gut Cell Atlas Roadmap to ensure accurate representation and full characterization of the cells of the human gut as part of the larger consortium. To ensure the successful assembly of this Atlas, coordinated research around a shared plan to address all critical aspects of gut biology, ensure reproducible findings, and avoid unnecessary duplication of effort will be provided. The Roadmap can be accessed here.
Team: Jennifer Rood, Ellen Todres
 “It is without question that those suffering from Crohn’s disease need better diagnostics and improved treatments. By better understanding which cells in the intestines express which genes, how these genes affect cell behavior, and which cells interact with each other, it will be possible to design new drugs and identify biomarkers to better stratify patients and improve their treatment outcomes.”
“It is without question that those suffering from Crohn’s disease need better diagnostics and improved treatments. By better understanding which cells in the intestines express which genes, how these genes affect cell behavior, and which cells interact with each other, it will be possible to design new drugs and identify biomarkers to better stratify patients and improve their treatment outcomes.”
The goal of this study is to build a detailed reference atlas of the cell types, and the genes they express, of the healthy terminal ileum and ascending colon, and a corresponding reference atlas of Crohn’s disease and use them to decipher the molecular basis of intestinal function and inflammatory disease processes. This grant will also identify characteristic molecular, cellular, and histological changes in disease that can be targeted therapeutically or serve as potential biomarkers.
Team: Kristin Ardlie, Evan Macosko, Fei Chen, Xiaowei Zhuang, Barbara Engelhardt, Sarah Teichmann, Carl Anderson, Matthias Zilbauer
Publications
The landscape of immune dysregulation in Crohn’s disease revealed through single-cell transcriptomic profiling in the ileum and colon. Immunity (2023).
Single-cell and spatial transcriptomics of stricturing Crohn’s disease highlights a fibrosis-associated network. Nature Genetics (2025).
The goal of this study is to conduct genomic and histologic analyses on patient samples and integrate these with data from the GCA consortium to better understand which cells contribute, and how, to the diversity and progression of Crohn’s disease. If successful, this study will help better understand genetic and histological signatures as they contribute to the pathogenesis and clinical diversity observed in Crohn’s disease, and shed insight into their potential use as biomarkers in clinical practice.
Team: Arkadiusz Gertych, Talin Haritunians, Dalin Li
Publications
Pre-operative visceral adipose tissue radiodensity is a potentially novel prognostic biomarker for early endoscopic post-operative recurrence in Crohn’s disease. World Journal of Gastrointestinal Surgery (2024).
 “There are several unresolved questions in intestinal stem cell biology, including whether there is a hierarchy of stem cells, what governs stem cell plasticity, and how stem cells survive in various injury and disease contexts. I am most excited about working with the Gut Cell Atlas consortium to address some of these unresolved questions.”
“There are several unresolved questions in intestinal stem cell biology, including whether there is a hierarchy of stem cells, what governs stem cell plasticity, and how stem cells survive in various injury and disease contexts. I am most excited about working with the Gut Cell Atlas consortium to address some of these unresolved questions.”
The goal of this study is to generate a comprehensive map of intestinal stem cells and their cellular signaling pathways in healthy versus Crohn’s disease biopsies from children and adults, which together with functional analyses of these cells, will support development of new strategies to heal the epithelial barrier in patients with Crohn’s disease. This grant will also define how these cell types differ between children and adults.
Team: Christopher Lengner, Judith Kelsen, Kai Tan, Meenakshi Bewtra
Publications
Unsupervised and supervised discovery of tissue cellular neighborhoods from cell phenotypes. Nature Methods (2024).
Isolation of Epithelial and Stromal Cells from Colon Tissues in Homeostasis and Under Inflammatory Conditions. Bio-protocol (2023).
An epigenetic basis for sustained inflammatory epithelial progenitor cell states in Crohn’s disease. CMGH (2025).
 “The Gut Cell Atlas is a bold endeavor that aims to understand the cells in the gut at the single-cell level, how their organization and interactions in the gut orchestrate tissue function, and what goes awry during Crohn's disease. The better we understand the individual components of the system, and how they work together, or cease to do so in disease, the better we're positioned to develop effective therapies.”
“The Gut Cell Atlas is a bold endeavor that aims to understand the cells in the gut at the single-cell level, how their organization and interactions in the gut orchestrate tissue function, and what goes awry during Crohn's disease. The better we understand the individual components of the system, and how they work together, or cease to do so in disease, the better we're positioned to develop effective therapies.”
The goal of this study is to develop a novel technology that enables spatially resolved high-throughput gene expression profiling of tissue sections, enabling efficient study of cell-to-cell communication mechanisms and identification of aberrations in the intestinal tissue of Crohn’s disease patients compared to healthy individuals. This grant will also provide the research community with an easily accessible tool to map spatial locations of gene transcripts within each intestinal cell and tissue.
“The last few years have brought unprecedented technical advances in the field of single cell genomics. These novel developments enable us to shed light on pathophysiology in disease areas with unmet medical needs.”
The goal of this study is to develop the sphere-sequencing method to systematically identify and rank cellular interactions that occur in the healthy intestine and within the Crohn’s disease microenvironment. This grant will also enable efficient study of cell-to-cell communication mechanisms by characterizing the genes expressed in each cell, the profiling of ligand – receptor pairs on neighboring cells and the corresponding spatial location of various cells.
Publications
Fragment-sequencing unveils local tissue microenvironments at single-cell resolution. Nature Communications (2023).
Stress-free single-cell transcriptomic profiling and functional genomics of murine eosinophils. Nature Protocols (2024).
“The Gut Cell Atlas, as well as the Human Cell Atlas, represent one of the forefronts of scientific research, as we try to understand complex biological systems by examining the activities and interactions of large numbers of individual cells. Studies at such a single-cell level will provide deeper understanding and generate new ideas for disease prevention and treatments.”
The goal of this study is to evaluate and validate the utility of a new computational method (dropout-based co-occurrence) of single-cell gene expression analysis for identifying cell types in the context of healthy and Crohn’s disease gut tissue. This grant will also allow for deeper understanding of the cell types in the ileum and how these cells are altered in Crohn’s disease, in patients of different ages and from different ancestry groups.
Team: Subra Kugathasan, Greg Gibson, Eliver Ghosn
Publications
Assessing Cellular and Transcriptional Diversity of Ileal Mucosa Among Treatment-Naïve and Treated Crohn's Disease. Inflammatory Bowel Diseases (2023).
Embracing the dropouts in single-cell RNA-seq analysis. Nature Communications (2020).
JSOM: Jointly-evolving self-organizing maps for alignment of biological datasets and identification of related clusters. PLOS Computational Biology (2021).
Human intestinal cell dissociation suitable for multi-omics single-cell assays. Protocols.io (2021).
 “Working on the Gut Cell Atlas will provide the opportunity to collect unprecedented scientific information on individual cells of the human gut both in health and in Crohn’s disease.”
“Working on the Gut Cell Atlas will provide the opportunity to collect unprecedented scientific information on individual cells of the human gut both in health and in Crohn’s disease.”
The goal of this study is to characterize enterochromaffin cells and resident macrophages, two cell populations heavily involved in pain perception and immune regulation, from the terminal ileum and ascending colon of healthy individuals and Crohn’s disease patients to identify critical pathways and biomarkers involved in pain signaling and modulation of the immune response triggered by signals from the nervous system. This grant will also map specific cell types and states to their spatial locations within preserved tissue architecture to infer and characterize networks of cell-cell interactions in health and Crohn’s disease.
Publications
Spatially resolved transcriptomic profiling of degraded and challenging fresh frozen samples. Nature Communications (2023).
Methods and applications for single-cell and spatial multi-omics. Nature Reviews Genetics (2023).
 The Ethics Working Group, supported by several funders, aims to enable rapid, international, and open-data sharing within the Human Cell Atlas and GCA communities while ensuring that generation and use of the data respect and protect participating individuals and patients. Harmonizing consent, privacy, security practices, access policies, and terms of use across the network will help ensure that data sharing proceeds in an effective and responsible manner and will contribute to the success of the initiative.
The Ethics Working Group, supported by several funders, aims to enable rapid, international, and open-data sharing within the Human Cell Atlas and GCA communities while ensuring that generation and use of the data respect and protect participating individuals and patients. Harmonizing consent, privacy, security practices, access policies, and terms of use across the network will help ensure that data sharing proceeds in an effective and responsible manner and will contribute to the success of the initiative.
Team: Emily Kirby, Alex Bernier
Publications
Human Cell Atlas Ethics Working Group.
Open Data in the Era of the GDPR: Lessons from the Human Cell Atlas. Annual Review of Genomics and Human Genetics Vol. 24 (August 2023).
 “Many complex diseases like Crohn’s disease, autism, allergies, diabetes are on the rise and as a result of our complex lifestyle. I am hopeful that the global concerted Human Cell Atlas initiative will continue to generate state-of-the-art tools and knowledge needed to understand such complex diseases and lead to treatments and prevention strategies.”
“Many complex diseases like Crohn’s disease, autism, allergies, diabetes are on the rise and as a result of our complex lifestyle. I am hopeful that the global concerted Human Cell Atlas initiative will continue to generate state-of-the-art tools and knowledge needed to understand such complex diseases and lead to treatments and prevention strategies.”
The goal of this study is to identify and characterize cell types and disease-specific gene expression patterns and regulatory features by establishing a comprehensive cellular atlas of the ileum and colon in healthy individuals and patients with ileal-colonic and ileal Crohn’s disease. This grant will also establish intestinal epithelial organoids and provide cell-specific gene expression and epigenetic data to understand how similar or different these models are from the cells in the body and help explain the mechanisms of Crohn’s disease.
Team: Eugene Chang, Sebastian Pott, Matthew Stephens
 The goal of this study is to establish a sample preparation, imaging, and algorithmic pipeline to create at a micro- and nano-scale reconstructions of all the cells and their characteristics for the human enteric system and other associated tissues. This grant will provide proof of concept of the applicability of the synchrotron source X-rays and high-resolution microscopy imaging modalities to human intestinal samples and further offer a valuable anatomical map for the GC community.
The goal of this study is to establish a sample preparation, imaging, and algorithmic pipeline to create at a micro- and nano-scale reconstructions of all the cells and their characteristics for the human enteric system and other associated tissues. This grant will provide proof of concept of the applicability of the synchrotron source X-rays and high-resolution microscopy imaging modalities to human intestinal samples and further offer a valuable anatomical map for the GC community.
Publications
Regional cytoarchitecture of the adult and developing mouse enteric nervous system. Current Biology (2022).
We will generate datasets that will be of use to researchers around the world for the benefit of Crohn’s disease patients
Mark Arends, University of Edinburgh
Working on the Gut Cell Atlas will provide the opportunity to collect unprecedented scientific information on individual cells of the human gut both in health and in Crohn’s disease.
Guy Boeckxstaens, Katholieke UniversiteitThe Gut Cell Atlas Crohn’s Disease Consortium is supported by the Helmsley Charitable Trust Crohn’s Disease Program.
It is a component of the Human Cell Atlas, which seeks to create comprehensive reference maps of all cells — the fundamental units of life — as a basis for both understanding human health and for diagnosing, monitoring, and treating disease.